Copyright August 2018
Research Experiment Summary:
Commercially available fresh and caramel apples were inoculated with Listeria monocytogenes. Wet Pre-Liminate™ Formula W31 containing probiotic organisms selected for competitive inhibition were added to fresh produce apples and caramel apples. The amount of probiotic added was achievable in common industry applications. All products were stored in their original packaging at ambient temperature (73°C) for the duration of the study. Multiple replications and repetitions/pathogen were analyzed using AOAC Method 2003.12 , a PCR-Dupont Qualicon BAX method and system. This study validates the use of competitive microorganisms can be considered as a valid and effective pathogen intervention for fresh and minimally processed fruits.
Results Summary:
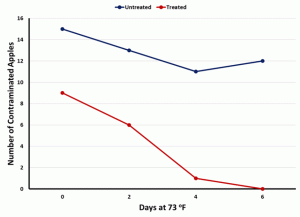 This research validates the use of a three-strain probiotic solution at specific concentrations, to control Listeria monocytogenes in fresh and caramel apples. Liquid sprays and/or dips are already used in fresh fruit and vegetable production. The concentration of probiotics, regardless of drying and heat affecting the counts, the dip was still successful over a 6-day, ambient temperature storage. The effectiveness was maintained with and without the application of caramel. Additional work performed with treated apples from production indicated no adverse effects on quality or sensory characteristics of the finished product. The application of the selected organisms did not detract from the quality of the apple but made it a higher-quality, probiotic delivery system that contained a lasting pathogen intervention system even after the sale of the product. This intervention may be extrapolated to other fresh, RTE and minimally processed fruits and vegetables like papaya, pears, cucumbers, tomatoes, etc. Additionally, it is important to note, other experiments in our laboratory show that these strains are also effective towards other pathogens like Salmonella, Clostridium, etc. So, not only is this a preventive control for Listeria, there may be additional latent protection from other potential contaminating pathogens.
This research validates the use of a three-strain probiotic solution at specific concentrations, to control Listeria monocytogenes in fresh and caramel apples. Liquid sprays and/or dips are already used in fresh fruit and vegetable production. The concentration of probiotics, regardless of drying and heat affecting the counts, the dip was still successful over a 6-day, ambient temperature storage. The effectiveness was maintained with and without the application of caramel. Additional work performed with treated apples from production indicated no adverse effects on quality or sensory characteristics of the finished product. The application of the selected organisms did not detract from the quality of the apple but made it a higher-quality, probiotic delivery system that contained a lasting pathogen intervention system even after the sale of the product. This intervention may be extrapolated to other fresh, RTE and minimally processed fruits and vegetables like papaya, pears, cucumbers, tomatoes, etc. Additionally, it is important to note, other experiments in our laboratory show that these strains are also effective towards other pathogens like Salmonella, Clostridium, etc. So, not only is this a preventive control for Listeria, there may be additional latent protection from other potential contaminating pathogens.
Probiotic Stability:
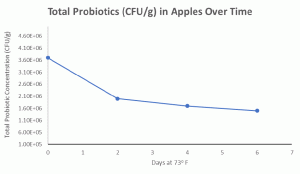 Storage data for probiotics demonstrate that the Lactobacillus and Bifidobacterium remained stable over storage with no adverse effects related to the quality of the inoculated apples. Further research is ongoing to determine the total length of time probiotic viability can be maintained.
Storage data for probiotics demonstrate that the Lactobacillus and Bifidobacterium remained stable over storage with no adverse effects related to the quality of the inoculated apples. Further research is ongoing to determine the total length of time probiotic viability can be maintained.
This report of original research by Log10® is the property of Log10®, LLC and is intended to provide information to clients of Log10®. It may not be copied or used either in part or in total without the permission of Log10®. Additional copies may be obtained by contacting Log10® at 580-304-7953.
